免疫學(xué),腫瘤免疫治療,體液免疫,抗體作用機(jī)制和優(yōu)化,B淋巴細(xì)胞發(fā)育分化
體液免疫與治療實(shí)驗(yàn)室的研究主要有兩個(gè)方向:(一)腫瘤免疫治療抗體作用規(guī)律和優(yōu)化策略;(二)B淋巴細(xì)胞的發(fā)育調(diào)控機(jī)制。
腫瘤免疫治療
腫瘤的免疫治療是過(guò)去十年取得的最有希望的科學(xué)進(jìn)步之一,被《科學(xué)》雜志評(píng)選為2013年“年度突破”。目前主要有四種手段可以利用免疫系統(tǒng)的力量來(lái)對(duì)抗腫瘤:1)針對(duì)腫瘤抗原的抗體被輸入到腫瘤病人體內(nèi)殺滅腫瘤細(xì)胞,其中部分機(jī)制是腫瘤細(xì)胞結(jié)合了抗體之后被表達(dá)Fc受體的免疫細(xì)胞殺滅;2)從腫瘤病人分離的抗腫瘤免疫細(xì)胞在體外擴(kuò)增(有時(shí)候還被基因修飾),然后回輸?shù)讲∪梭w內(nèi)發(fā)揮抗腫瘤作用;3)T細(xì)胞被基因工程修飾后表達(dá)識(shí)別腫瘤抗原的嵌合抗原受體(Chimeric Antigen Receptor,CAR)被輸入到腫瘤病人體內(nèi);4)利用單克隆抗體(單抗)阻斷限制免疫細(xì)胞活性的免疫抑制信號(hào)通路(被稱為“節(jié)點(diǎn)”)從而提高免疫細(xì)胞的活性。
激動(dòng)型抗體:一類具有廣泛前景的腫瘤免疫治療抗體
體液免疫與治療實(shí)驗(yàn)室的一個(gè)主要研究興趣是激動(dòng)型腫瘤免疫治療抗體的作用規(guī)律和優(yōu)化策略。已經(jīng)研發(fā)成功的腫瘤免疫治療藥物包括抗CTLA-4與抗PD-1/PD-L1 抗體,它們都通過(guò)阻斷免疫細(xì)胞表面?zhèn)鬟f免疫抑制信號(hào)的分子的作用增強(qiáng)免疫系統(tǒng)針對(duì)腫瘤細(xì)胞產(chǎn)生的殺傷性T細(xì)胞應(yīng)答從而殺傷腫瘤,屬于阻斷型抗體。而除此之外,還有一類被稱為“激動(dòng)型抗體”的腫瘤免疫治療手段,能夠通過(guò)結(jié)合免疫細(xì)胞表面?zhèn)鬟f免疫激活信號(hào)的靶標(biāo)分子并激活其控制的重要免疫激活信號(hào)通路,進(jìn)而增強(qiáng)抗腫瘤免疫應(yīng)答間接殺死腫瘤細(xì)胞。然而,雖然激動(dòng)型腫瘤免疫治療抗體已經(jīng)在動(dòng)物模型中證明了其巨大潛力,并且已經(jīng)成為一個(gè)被廣泛接受并看好的腫瘤免疫治療理念,這類抗體的研發(fā)至今尚未成功,是腫瘤免疫治療領(lǐng)域當(dāng)前的一個(gè)主要挑戰(zhàn)。糾其原因,可能與這類抗體的體內(nèi)作用條件不明有關(guān)。在基于鼠類激動(dòng)型抗腫瘤抗體的研究中,我們發(fā)現(xiàn)了一條可能有助于研發(fā)具有較好抗腫瘤活性的激動(dòng)型抗體的線索,即這些抗體的Fcγ受體(Fcγ receptor,或FcγR)結(jié)合能力對(duì)它們的抗腫瘤活性具有決定性的影響,現(xiàn)在正在展開(kāi)研究。
Fcγ受體的體液免疫與治療調(diào)控功能
Fcγ受體與IgG抗體相互作用,是IgG抗體治療作用的重要介導(dǎo)者和調(diào)控者;同時(shí),F(xiàn)cγ受體與含有IgG的免疫復(fù)合物相互作用,調(diào)控免疫應(yīng)答的傳入和傳出,如對(duì)B細(xì)胞應(yīng)答的調(diào)控。Fcγ受體廣泛表達(dá)在免疫細(xì)胞表面,介導(dǎo)抗體的免疫調(diào)控和效應(yīng)功能。人和小鼠的FcγR家族都由幾個(gè)活化性FcγRs(activating FcγRs)和一個(gè)抑制性Fcγ受體(Inhibitory FcγRIIB,或FcγRIIB)組成(圖1)。抑制性Fcγ受體胞內(nèi)區(qū)含有酪氨酸依賴的抑制型基序(Immunoreceptor Tyrosine-based Inhibitory Motif,或ITIM)傳遞抑制信號(hào),起抑制細(xì)胞激活的作用。活化性FcγRs通過(guò)含有酪氨酸依賴的激活型基序(Immunoreceptor Tyrosine-based Activation Motif,或ITAM)的胞內(nèi)區(qū)或Fc受體γ鏈(Fc receptor γ chain)傳遞活化信號(hào),促進(jìn)細(xì)胞激活。大量的實(shí)驗(yàn)已經(jīng)證明活化性FcγRs在IgG抗體的效應(yīng)功能中發(fā)揮主導(dǎo)作用。例如,抗體依賴細(xì)胞介導(dǎo)的細(xì)胞毒性作用(Antibody-Dependent Cell-Mediated Cytotoxicity,或ADCC)就是通過(guò)活化性FcγRs完成的。ADCC是效應(yīng)型治療抗體重要的腫瘤殺傷機(jī)制,對(duì)這類抗體(如Rituximab)的療效有顯著影響。
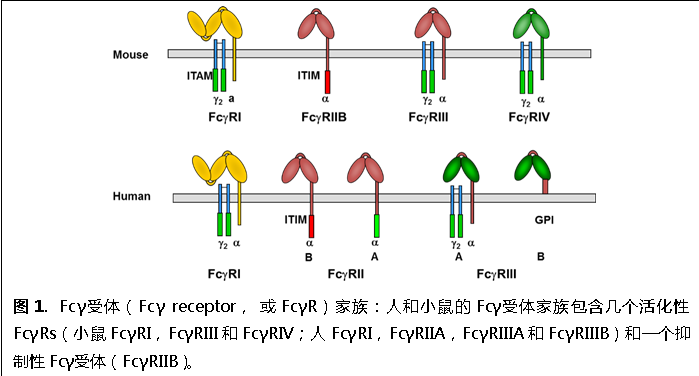
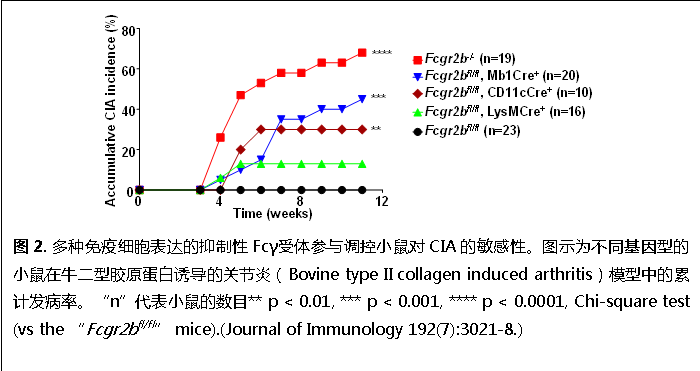
我們發(fā)現(xiàn)抑制性Fcγ受體能夠?qū)Χ喾N免疫細(xì)胞的調(diào)控對(duì)免疫耐受的維持都具有顯著影響(Li et al. 2014. Journal of Immunology 192(7):3021-8.),如在B細(xì)胞與樹(shù)突狀細(xì)胞(dendritic cells)中特異敲除抑制性Fcγ受體致使小鼠在CIA(collagen induced arthritis)自身免疫模型中更加敏感(圖2).此外,人類抑制性Fcγ受體的功能受損被發(fā)現(xiàn)與包括系統(tǒng)性紅斑狼瘡、風(fēng)濕性關(guān)節(jié)炎等多種自身免疫疾病。
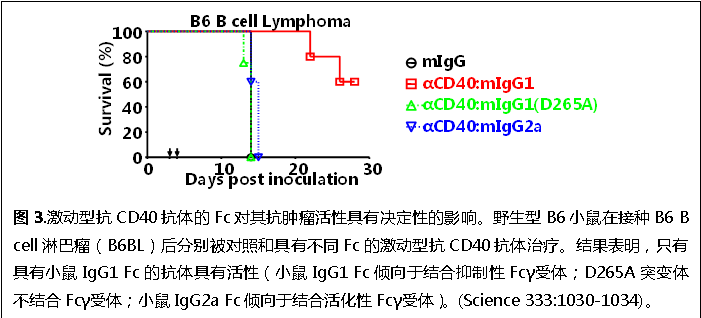
我們還系統(tǒng)研究了Fc-FcγRs相互作用對(duì)激動(dòng)型抗TNF受體超家族成員抗體(agonistic anti-TNFR antibodies)分子的體內(nèi)活性的影響。以激動(dòng)型抗CD40和DR5抗體為例,我們發(fā)現(xiàn)這類抗體的體內(nèi)活性依賴于Fcγ受體分子,而且抗體Fc的屬性對(duì)其體內(nèi)抗腫瘤活性具有決定性的影響(圖3)。
進(jìn)一步研究表明,與依賴于活化性Fcγ受體的效應(yīng)型抗體不同,激動(dòng)型抗TNFR抗體體內(nèi)活性只依賴于抑制性Fcγ受體,而與活化性FcγRs的相互作用會(huì)降低其活性。在此基礎(chǔ)上,我們提出了一種優(yōu)化激動(dòng)型抗TNFR超家族成員抗體的方法(圖4)
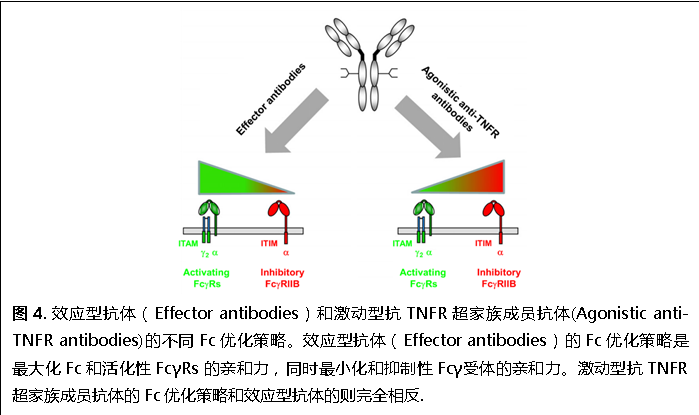
:最大化與抑制性Fcγ受體的結(jié)合親和力,同時(shí)最小化與活化性FcγRs的結(jié)合親和力,和傳統(tǒng)的優(yōu)化效應(yīng)型抗體的方法剛好相反。用這種方法優(yōu)化的人抗小鼠CD40抗體都已經(jīng)在人抑制性Fcγ受體轉(zhuǎn)基因小鼠模型中得到了驗(yàn)證(圖5)。
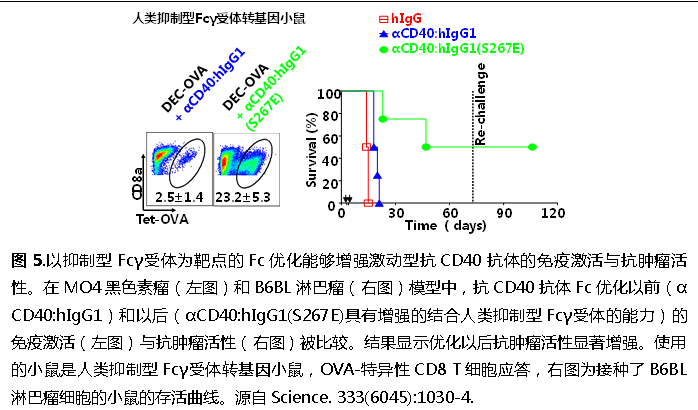
這些成果被連續(xù)發(fā)表在包括2011年Science(Li, F., and J.V. Ravetch. 2011. Science 333:1030-1034),2012年P(guān)NAS雜志(Li, F., and J.V. Ravetch. 2012. Proceedings of the National Academy of Sciences of the United States of America 109:10966-71),和2013年P(guān)NAS雜志(Li, F., and J.V. Ravetch. 2013. Proceedings of the National Academy of Sciences of the United States of America 110:19501-6),和2016年Cancer Cell雜志(Dahan, R., B. C. Barnhart, F. Li, A. P. Yamniuk, A. Korman, J.V. Ravetch. 2016. Cancer Cell),并受邀在Cell Cycle雜志上就這一研究領(lǐng)域的進(jìn)展發(fā)表評(píng)論文章(Li, F., and J.V. Ravetch. 2012. Cell cycle 11:18)。這些研究結(jié)果為解釋尚未成功的激動(dòng)型抗TNFR抗體研發(fā)提供了一條線索,可能有利于研發(fā)基于這類抗體的抗腫瘤藥物。
目前,實(shí)驗(yàn)室工作的重點(diǎn)是進(jìn)一步研究腫瘤免疫治療抗體作用規(guī)律和優(yōu)化策略,包括Fcγ受體相關(guān)和其它的調(diào)控規(guī)律和策略;同時(shí),實(shí)驗(yàn)室對(duì)B淋巴細(xì)胞的發(fā)育調(diào)控規(guī)律也非常感興趣,正在開(kāi)展Fcγ受體及相關(guān)分子對(duì)體液免疫的調(diào)控機(jī)制。
1. Li, M., Lazorchak, A.S., Ouyang, X., Zhang, H., Liu, H., Arojo, O.A., Yan, L., Jin, J., Han, Y., Qu, G., Xu, X., Liu, X., Zhang, W., Wang, Q., Liu, D., Li, F.#, Su, B., .Sin1/mTORC2 regulates B cell growth and metabolism via mTORC1 and Myc activation..Cellular & Molecular Immunology,2019,16(9):757-769. [Link]
2. Wenqian Zhang, Huihui Zhang, Shujun Liu, Fucan Xia, Zijian Kang, Yan Zhang, Yaoyang Liu, Hui Xiao, Lei Chen, Chuanxin Huang, Nan Shen, Huji Xu, and Fubin Li.Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus.PNAS,2019,116 (37) 18550-18560. [Link]
3. Xiaobo Liu, Yingjie Zhao, Huan Shi, Yan Zhang, Xueying Yin, Mingdong Liu, Huihui Zhang, Yongning He, Boxun Lu, Tengchuan Jin & Fubin Li.Human immunoglobulin G hinge regulates agonistic anti-CD40 immunostimulatory and antitumor activities through biophysical flexibility.Nature Communications,2019,4206. [Link]
4. Dahan, R., B. C. Barnhart, F. Li, A. P. Yamniuk, A. Korman, J.V. Ravetch. .Therapeutic activity of agonistic, human anti-CD40 monoclonal Abs requires ive FcγR-engagement. .Cancer Cell ,2016,29(6):820-831. [Link]
5. Georgoudaki, A.M., K. Prokopec, E. Hellqvist, V. Boura, J. Östling, S. Sohn, R.A. Harris, M. Rantalainen, D. Klevebring, M. Sund, J. Fuxe, C. Rolny, F. Li, J.V. Ravetch, M.C.I. Karlsson. .Reprogramming tumor associated macrophages by antibody targeting inhibits cancer progression and metastasis. .Cell Reports ,2016,15(9):2000-11. [Link]
6. Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, Shi H, Liu M, Du M, Taylor PR, Zhu HH, Chen J, Meng G, Li F, Chen C, Zhang Y, Jia XM, Lin X, Zhang X, Pearlman E, Li X, Feng GS, Xiao H. .Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses..Nature Immunology,2015,16:642-52. [Link]
7. Li, F#., P. Smith, and J. V. Ravetch#. .Inhibitory Fcgamma Receptor Is Required for the Maintenance of Tolerance through Distinct Mechanisms. .Journal of immunology ,2014,192:3021-8 . [Link]
8. Simpson, T. R., F. Li, W. Montalvo-Ortiz, M. A. Sepulveda, K. Bergerhoff, F. .Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma.The Journal of experimental medicine,2013,210: 1695-1710. [Link]
9. Li, F#., and J. V. Ravetch#. .Antitumor activities of agonistic anti-TNFR antibodies require differential FcgammaRIIB coengagement in vivo. .Proceedings of the National Academy of Sciences of the United States of America,2013,110: 19501-19506. [Link]
10. Smith, P., D.J. Dilillo, S. Bournazos, F. Li, and J.V. Ravetch.Mouse model recapitulating human Fcgamma receptor structural and functional diversity. .Proceedings of the National Academy of Sciences of the United States of America ,2012,109:6181-6186. [Link]
11. Li, F., and J. V. Ravetch.Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcgamma receptor engagement..Proceedings of the National Academy of Sciences of the United States of America ,2012,109: 10966-10971. [Link]
12. Li, F., and J. V. Ravetch.A general requirement for FcgammaRIIB co-engagement of agonistic anti-TNFR antibodies..Cell cycle ,2012,11: 3343-3344. [Link]
13. Li, F., and J.V. Ravetch.Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. .Science ,2011,333:1030-1034. [Link]
14. Li, F., Y. Yan, J. Pieretti, D.A. Feldman, and L.A. Eckhardt. .Comparison of identical and functional Igh alleles reveals a nonessential role for Emu in somatic hypermutation and class-switch recombination. .Journal of immunology ,2010,185:6049-6057. [Link]
15. Li, F., and L.A. Eckhardt.A role for the IgH intronic enhancer E mu in enforcing allelic exclusion.The Journal of experimental medicine ,2009,206:153-167. [Link]
16. Zhang, B., A. Alaie-Petrillo, M. Kon, F. Li, and L.A. Eckhardt.Transcription of a productively rearranged Ig VDJC alpha does not require the presence of HS4 in the IgH 3 regulatory region. .Journal of immunology ,2007,178:6297-6306. [Link]
17. Romov, P.A., F. Li, P.N. Lipke, S.L. Epstein, and W.G. Qiu..Comparative genomics reveals long, evolutionarily conserved, low-complexity islands in yeast proteins. .Journal of molecular evolution,2006,63:415-425. [Link]
18. Garrett, F.E., A.V. Emelyanov, M.A. Sepulveda, P. Flanagan, S. Volpi, F. Li, D. Loukinov, L.A. Eckhardt, V.V. Lobanenkov, and B.K. Birshtein.Chromatin architecture near a potential 3 end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites.Molecular and cellular biology,2005,25:1511-1525. [Link]
 滬公網(wǎng)安備 31009102000053號(hào) 滬ICP備18007527號(hào)-1 郵箱:[email protected]
滬公網(wǎng)安備 31009102000053號(hào) 滬ICP備18007527號(hào)-1 郵箱:[email protected]



